| STANNOUS OXALATE | |||
|
PRODUCT IDENTIFICATION |
|||
| CAS NO. | 814-94-8, 17480-26-1 |
| |
| EINECS NO. | 212-414-0 | ||
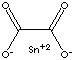
| FORMULA |
SnC2O4 | ||
| MOL WT. |
206.71 | ||
|
H.S.CODE |
2915.90 | ||
| TOXICITY | |||
| SYNONYMS | Tin (II) Oxalate; Oxalic acid tin salt; | ||
| Zinn(II)oxalat (German); Oxalato de estaño(II) (Spanish); Oxalate d'étain(II) (French); | |||
| SMILES | |||
|
CLASSIFICATION |
|
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
|||
| PHYSICAL STATE |
white powder | ||
| MELTING POINT | |||
| BOILING POINT | |||
| SPECIFIC GRAVITY | |||
| SOLUBILITY IN WATER | |||
| pH | |||
| VAPOR DENSITY | |||
AUTOIGNITION |
| ||
NFPA RATINGS |
| ||
REFRACTIVE INDEX |
| ||
| FLASH POINT |
| ||
| STABILITY | Stable under ordinary conditions | ||
|
GENERAL DESCRIPTION OF OXALIC ACID (OXALATE) & APPLICATIONS |
|||
|
Application:Oxalic Acid (also called Ethanedioic Acid) is a colourless, crystalline, toxic organic compound belonging to the family of dicarboxylic acids; melting at 187 C; soluble in water, alcohol, and ether. It occurs in the form of its metal salts (usually calcium or potassium) in many plants. It is commercially manufactured by heating sodium formate in the presence of an alkali catalyst to form sodium oxalate, which should be converted to free oxalic acid when treated with sulfuric acid. It is also prepared by oxidizing carbohydrates with nitric acid, by heating saw dust with caustic alkalies or by fermentation of sugar solutions in the presence of certain molds. Oxalic acid is the only possible compound in which two carboxyl groups are joined directly; for this reason oxalic acid is one of the strongest acids in organic compounds. Unlike other carboxylic acids, oxalic acid (and formic acid) is readily oxidized and combine with calcium, iron, sodium, magnesium, or potassium to form less soluble salts called oxalates. Oxalic acid and oxalates are useful as reducing agents for photography, bleaching, and rust removal. They are widely used as an purifying agent in pharmaceutical industry, precipitating agent in rare-earth metal processing, bleaching agent in textile and wood industry, rust-remover for metal treatment, grinding agent, waste water treatment. acid rinse in laundries and removing scale from automobile radiators. Stannous Oxalate is used as a catalyst (Esterification reactions). |
|||
| SALES SPECIFICATION | |||
|
APPEARANCE |
white powder | ||
|
SnC2O4 |
98.0% min | ||
| Cl | 0.1% max | ||
| Fe | 10ppm max | ||
|
Pb |
100ppm max | ||
| Sn |
10ppm max | ||
| TRANSPORTATION | |||
| PACKING | 25kgs in Bag | ||
| HAZARD CLASS | Not Regualted | ||
| UN NO. |
| ||
| OTHER INFORMATION | |||
| Hazard Symbols: XN, Risk Phrases: 21/22, Safety Phrases: 24/25 | |||
| DESCRIPTION OF TIN COMPOUNDS | |||
|
Tin compounds are classified into two main groups; inorganic-tin and organo-tin compounds. The organo-tin compounds are defined as compounds in which at least one tin-to-carbon bond exist. But the inorganic-tin compounds do not contain carbon as the principal element. Inorganic-tin compounds are relatively simple in their molecular structure and, like tin itself, are not considered to be toxic. Tin atoms can replace carbon atoms in chemical compounds, and a great variety of organo-tin compounds are known. INORGANIC TIN COMPOUNDS The largest use for inorganic tin compounds is in electrolytes for plating tin and tin alloys. The more important plating chemicals are chlorides, sulfates, and fluoroborates in acidic electrolytes and stannates in alkaline solutions. Inorganic-tin compounds are divided into two series: stannous, or tin(II), compounds and stannic, or tin(IV), compounds.Chemically, tin exhibits valencies of 2 and 4. It resists attack by water but is dissolved by strong acids and alkalis. One of common compounds of tin(II) are stannous chloride (SnCl2) used in tin galvanizing, as a reducing agent in the manufacture of polymers and as a mordant in dyeing.; stannous oxide (SnO) employed in making tin salts for chemical reagents and for plating; and stannous fluoride (SnF2) is the additive in fluoride tooth-pastes. Inorganic tin chemicals are used as catalysts in a number of industrial processes. stannous octoate is the catalyst that produces the foaming action that turns the liquid plastic into a foamlike solid structure in the manufacture of polyurethane foam. Tin(IV) compounds of significance include stannic chloride (SnCl4) is widely used as a stabilizer for perfumes and as a starting material for other tin salts; and stannic oxide(SnO2) is a useful catalyst in certain industrial processes and a polishing powder for steel. Tin sulfide is used as a bronzing agent for wood colouring ORGANOTIN COMPOUNDS The greatest use of di-organotin compounds is stabilizers in the
manufacture of polyvinyl chloride, or PVC. The particular importance of these
di-organotins lies in their outstanding ability to preserve the clarity and
transparency of PVC, not only when being processed but also in subsequent
service. Organotin-stabilized PVC is used in water pipes and in food packaging
applications as tin compounds used in these applications are known as nontoxic.
In contrast to the nontoxic compounds employed as stabilizers, some
tri-organotin compounds (e.g., tributyl- and triphenyltins) are powerful
biocides and have found use in a number of relevant applications, such as
fungicide, algicide, molluscicide in underwater and anti-fouling paints
extensively, preservatives for wood, as slimicide on masonry, as biocide
disinfectant for textile and leather processing, cooling system, pulp and paper
mill and brewery. The tributyltin family or fentine (triphenyltin) chemicals
include;
Tributyltin compounds are usually clear to yellowish liquids with an unpleasant odor. Triphenyltincompounds are white solids with low vapour pressures. Tri-organotin compounds are derivatives of tetravalent tin. They are lipophilic and have low water solubility. Physical and chemical properties of tri-organotin compounds vary depending upon the anion linked to tin. Tributyltin derivatives have toxic properties to gram positive bacteria are used as disinfectants on surfaces such as hospital floors and sports arenas, combined with gram negative bactericides. Tin chemicals also used as flame retardants to treat fabrics and plastics. Tributyltin methacrylate is used as a stabiliser for PVC. Other industrial applications of organotin compounds include as rodent repellents, antioxidants, curing agents and corrosion inhibitors. |
|||
| PRICE INFORMATION | |||